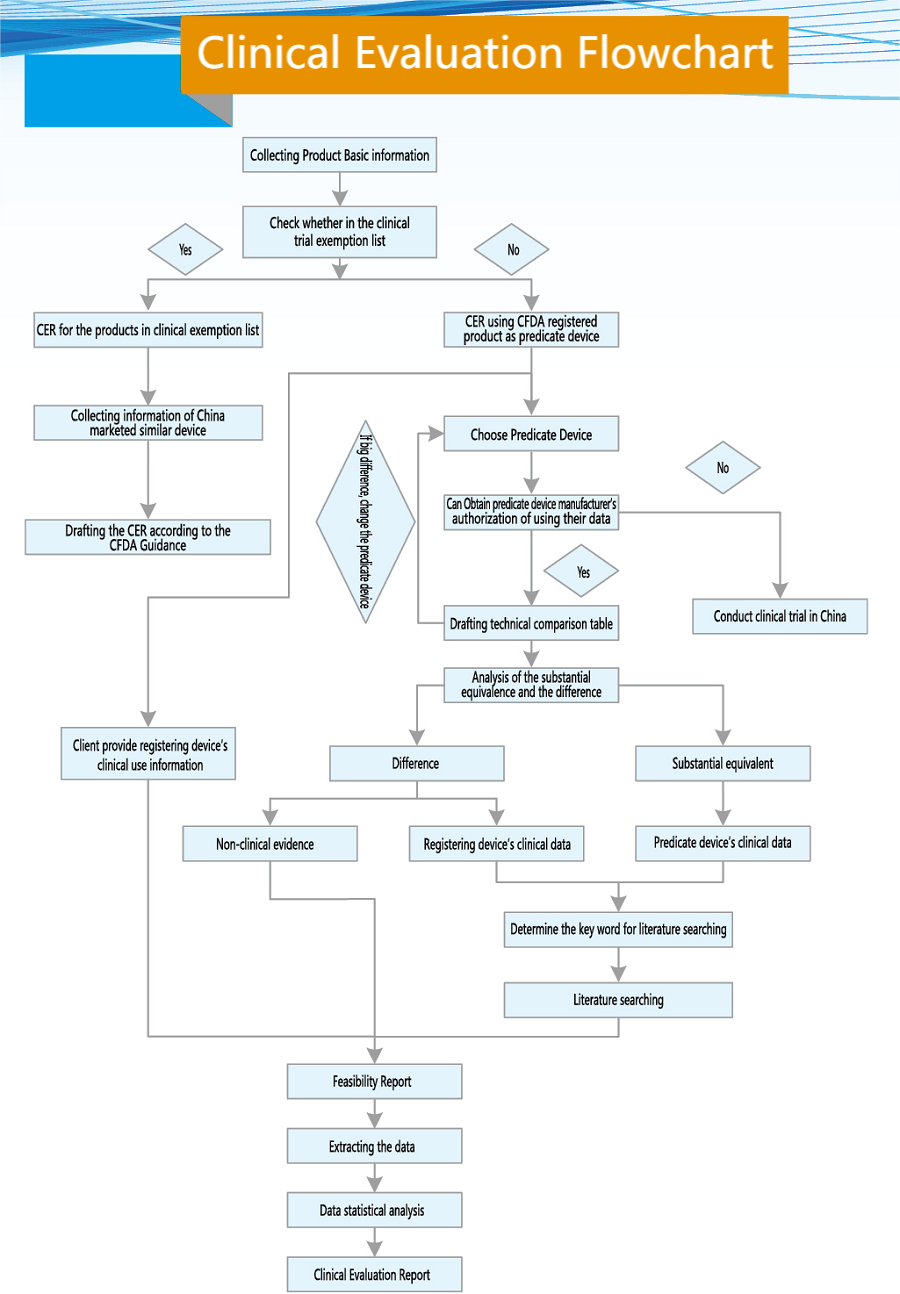
Definition of clinical evaluation
Medical device clinical evaluation refers to the process during which the applicant verifies that the register product meets the requirements of use or applications through summarizing the data from clinical literature, clinical experience, and clinical trials, etc.
lDesired conclusions of CER
lUnder normal conditions of use, the register product can achieve the desired performance
lThe risks of the register product are acceptable in comparison with the expected benefits
lThe clinical performance and safety of the register product can be appropriately supported
Principle of clinical evaluation
Clinical evaluations should be comprehensive and objective The depth and breadth of clinical evaluations as well as required data types and quantities should match with register product’s design features, key technologies, applications and degree of risks, and should correspond to the level and extent of non-clinical studies.